- Ajou GSIS
- Academics
- Admissions
-
Student Life
Student Life
- Alumni
-
Notice
Notice
Notice
Ajou Univ. News
NEW A Research Team Led by Profs. Seo and Kim Uses Methane to Develop Green Hydrogen and Carbon Technologies
- 2021-09-06
- 3213

A joint research team from Ajou University has developed a catalyst response technology that can produce hydrogen and carbon using methane, the main component of city gas, or natural gas. This is a cutting-edge energy technology that can concurrently mass produce green, affordable hydrogen fuel and high purity, high value-added carbon.
The joint research team, led by Prof. Seo Hyung-tak (Dept. of Materials Science and Engineering, Graduate School Department of Energy Systems Research) and Prof. Kim Yu-kwon (Chemistry, Graduate School Department of Energy Systems Research) announced the development of technology to produce hydrogen gas and solid carbon directly converted from methane using a bubble column reactor injected with molten liquid alloy catalysts and zirconia. Their achievement was published in the online edition of Chemical Engineering Journal (IF=13.273) on July 7, 2021 under the title, “Enhanced Efficiency in CO2-free Hydrogen Production from Methane in a Molten Liquid Alloy Bubble Column Reactor with Zirconia Beads.” Lee Young-jae, a doctoral student at Ajou University, and Dr. Noh Yong-gyu took part as first co-authors, and another doctoral student, Kim Jin-a, and Prof. Shankara Kalanur also joined.
Hydrogen, a clean fuel that emits water after use, has recently gained recognition as a next-generation energy source and is increasingly applied to diverse industrial fields. Mass production of hydrogen as fuel is commonly achieved by reforming fossil fuels, but the process emits CO2 that is nine times greater than the weight of hydrogen thereby produced.
Due to this and other reasons, research has recently been active in photoelectrochemical water splitting technology using electricity or solar energy towards reducing emission of CO2, a major greenhouse gas. However, barriers have existed to these efforts as the unit cost of production has been at least five to six times the cost of reforming fossil fuels, and it could not be mass produced. Therefore, development of a way to mass produce hydrogen in an affordable and CO2-free manner is urgently needed if we are to realize a hydrogen economy, with hydrogen energy as a completely clean fuel.
The Ajou University research team focused on methane gas (CH4), a major component in city and natural gas. When methane gas undergoes a pyrolysis process using solid catalysts at temperatures greater than 1000℃, it produces hydrogen gas and solid carbon. The solid carbon thereby produced could not be commercialized previously as it becomes deactivated. When solid carbon accumulates on the surface of the catalyst, it ultimately prevents the chemical reaction of methane gas there, as a result of which its reaction activity rapidly disappears.
To address this limitation, researchers have recently turned their attention to the use of low-melting liquid metal (LM) alloy catalysts (liquid metal catalyst combining low-melting metals, such as tin, and high-melting metals, such as nickel, which are high catalyst activation). In other words, direct molten catalyst methane conversion technology injects methane gas into molten liquid metal alloy to produce hydrogen. At the same time, flotation and reaction are maintained due to the difference in carbon layer density on the surface of the liquid catalyst while also producing solid carbon.
The joint research team developed a new method, based on this direct molten catalyst methane conversion technology, to discover that maximizing the surface area of the boundary between methane gas and liquid catalyst by reducing the residence time of methane gas inside the liquid catalyst and size of the methane gas bubble in the methane decomposition reaction using liquid catalysts is a critical factor in enhancing reaction efficiency. The research team developed a new reactor structure by additionally injecting zirconia (a mixture of zirconium and oxygen (ZrO2) which has a high melting point, is not easily decomposed, and is in a white crystalline form at room temperature) to the liquid catalyst in the reactor to minimize the size of the methane bubble and complicate the gas flow channel, which increased gas residence time.
The adoption of a new reactor structure resulted in a high hydrogen conversion rate (about 37% methane at 985℃, lower than the existing temperature of at least 1000℃), and strong reaction durability as catalyst activity increased even after a long production period of more than 150 hours. In general, methane thermal decomposition reaction at 1000℃ or lower lowers the cost of thermal energy in accordance with reactor quality, but it also resulted in a rapid decrease in conversion rate. The Ajou research team overcame this stumbling block to record the highest conversion rate and continuous reaction at low temperature.
The carbon produced using this new method was in a form that is high purity and high value-added (carbon nanotube and fiber filament). Such carbon can be used in batteries and fuel cells. This new development taps into the potential not only for environmentally-friendly mass production of hydrogen but also high-applicability carbon materials.
“The existing hydrogen production methods using fossil fuels will inevitably undergo a rise in price due to trends toward stronger regulations on CO2 emissions,” said Prof. Seo. “This research is significant in that it has opened up a new green and affordable path of mass production of hydrogen.”
He also added, “It can be remarkably economical if the carbon produced simultaneously and continuously with hydrogen is of high purity and has high value-added properties. We will continue our research and aim for its commercialization.”
The research was sponsored by the Industrial Technology Alchemist Project managed by the Korean Ministry of Trade, Industry, and Energy (MOTIE), Korea Evaluation Institute of Industrial Technology (KEIT) and C1 Gas Refinery R&D Center managed by the Korean Ministry of Science and ICT and the National Research Foundation of Korea (NRF).
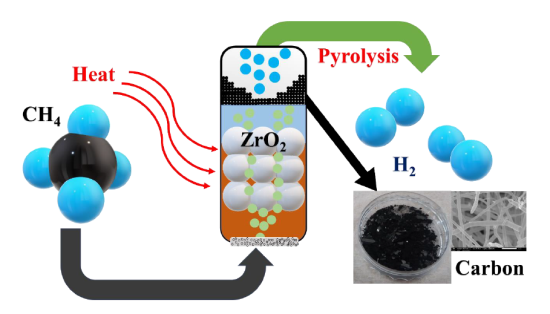
This is a concept diagram of the production of hydrogen and carbon using direct methane decomposition based on molten liquid alloy catalysts: when methane is injected into the reactor, which is mixed with liquid metal alloy catalyst and zirconia, and heated, high purity hydrogen forms inside the liquid catalyst, while at the same time creating carbon fiber. Carbon fiber is of low density and will float to the surface. This is why it is not affected by the catalytic reaction and long hours of continuous production of hydrogen and carbon are possible.
*Photo
From far left: Prof. Seo Hyung-tak, PhD students Lee Young-jae and Park Jin-a, and Prof. Kim Yu-kwon
 아주대학교 국제대학원
아주대학교 국제대학원